TDP#1 Probes for High Resolution Mechanobiology
The goal of this TDP is to transform the research accessibility of DNA molecular tension probes (MTP) and advance its capabilities, allowing orders of magnitude improvement in probe stability, spatial resolution, and orientational resolution. The work will help broaden the accessibility of DNA MTP to the research community and enable new biomedical applications, aligning with the long-term goals of the BTDD.
Super Resolution Tension Imaging (Tension PAINT)
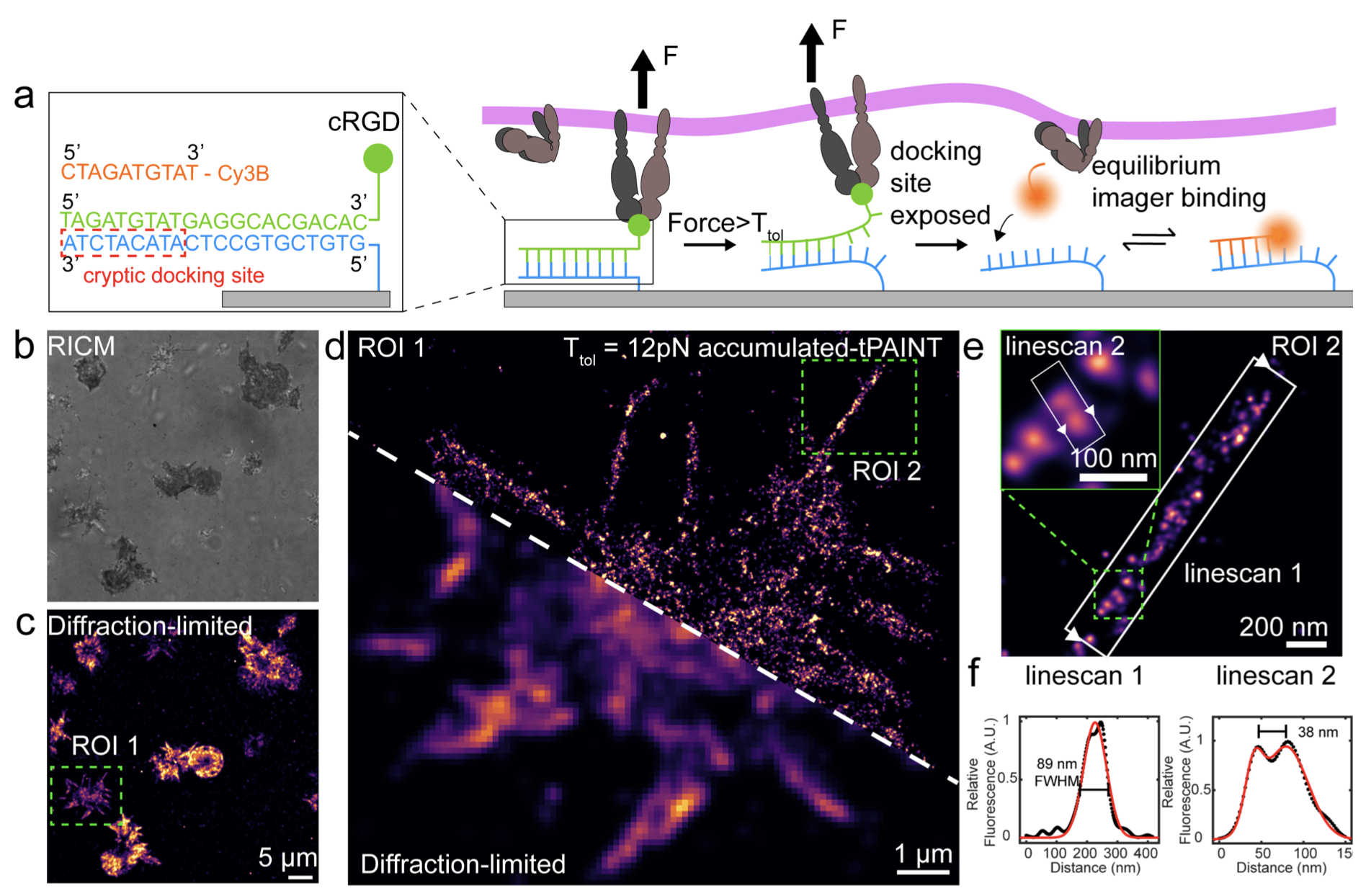
Despite the vital role of mechanical forces in biology, it still remains a challenge to image cellular force with sub-100-nm resolution. Here, we present tension points accumulation for imaging in nanoscale topography (tPAINT), integrating molecular tension probes with the DNA points accumulation for imaging in nanoscale topography (DNA-PAINT) technique to map piconewton mechanical events with ~25-nm resolution. To perform live-cell dynamic tension imaging, we engineered reversible probes with a cryptic docking site revealed only when the probe experiences forces exceeding a defined mechanical threshold (~7–21 pN). Additionally, we report a second type of irreversible tPAINT probe that exposes its cryptic docking site permanently and thus integrates force history over time, offering improved spatial resolution in exchange for temporal dynamics. We applied both types of tPAINT probes to map integrin receptor forces in live human platelets and mouse embryonic fibroblasts. Importantly, tPAINT revealed a link between platelet forces at the leading edge of cells and the dynamic actin-rich ring nucleated by the Arp2/3 complex.
Adapted from Brockman & Su et al. Nature Methods 2020.
Mapping the 3D Orientation of Piconewton Traction Force (MFM)
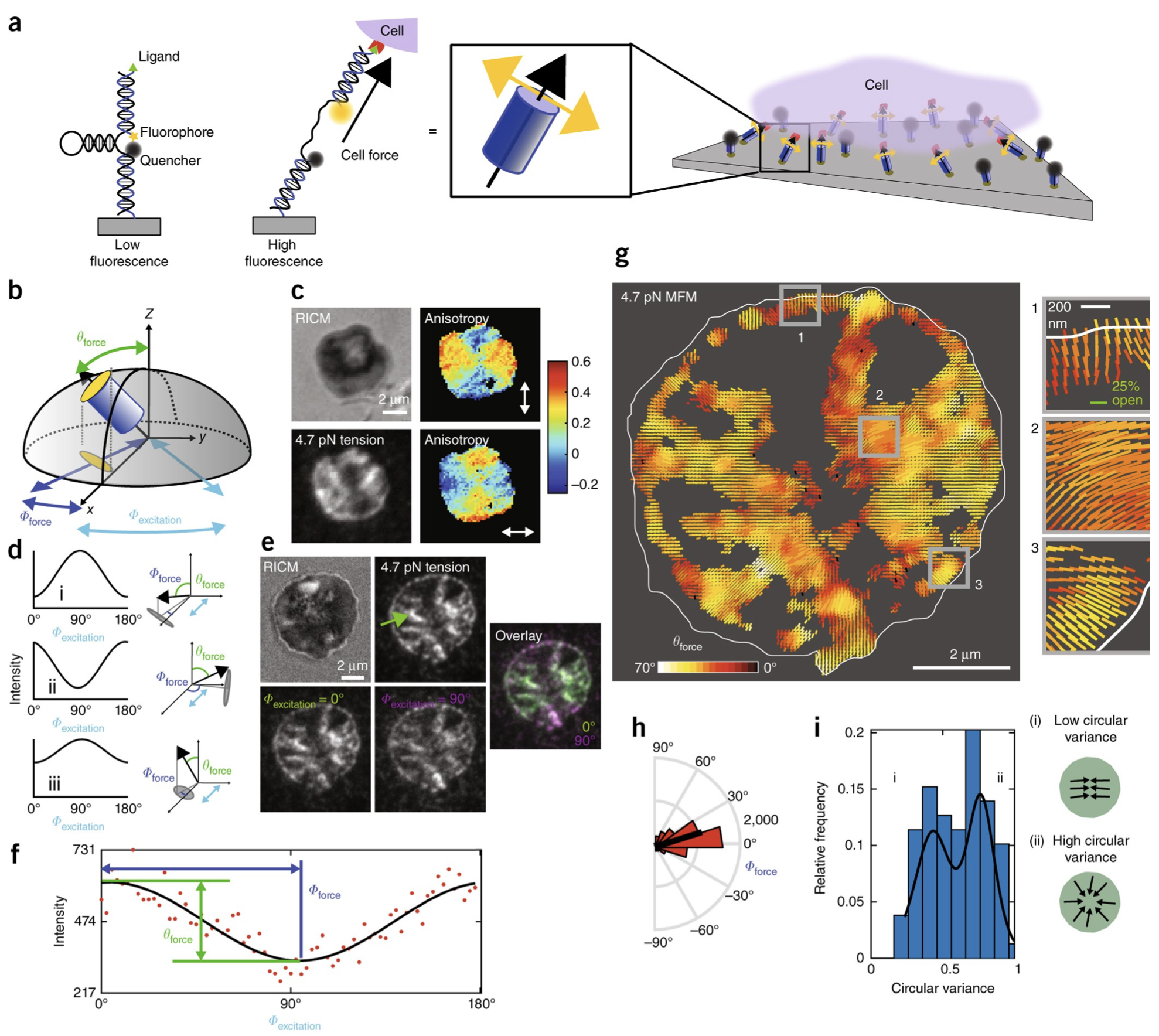
Mechanical forces are integral to many biological processes; however, current techniques cannot map the magnitude and direction of piconewton molecular forces. Here, we describe molecular force microscopy, leveraging molecular tension probes and fluorescence polarization microscopy to measure the magnitude and 3D orientation of cellular forces. We mapped the orientation of integrin-based traction forces in mouse fibroblasts and human platelets, revealing alignment between the organization of force-bearing structures and their force orientations.
Adapted from Brockman & Blanchard et al. Nature Methods 2018.
Mapping of Traction Force Orientation and Magnitude using Structured Illumination Microscope (SIM-MFM)
Many cellular processes, including cell division, development, and cell migration require spatially and temporally coordinated forces transduced by cell-surface receptors. Nucleic acid-based molecular tension probes allow one to visualize the piconewton (pN) forces applied by these receptors. Building on this technology, we recently developed molecular force microscopy (MFM) which uses fluorescence polarization to map receptor force orientation with diffraction-limited resolution (~250 nm). Here, we show that structured illumination microscopy (SIM), a super-resolution technique, can be used to perform super-resolution MFM. Using SIM-MFM, we generate the highest resolution maps of both the magnitude and orientation of the pN traction forces applied by cells. We apply SIM-MFM to map platelet and fibroblast integrin forces, as well as T cell receptor forces. Using SIM-MFM, we show that platelet traction force alignment occurs on a longer timescale than adhesion. Importantly, SIM-MFM can be implemented on any standard SIM microscope without hardware modifications.
Adapted from Blanchard et al. Nat Commun 2021